The gas hydrazine, , burns in oxygen like this:
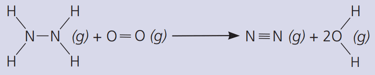
Calculate the total energy released when the new bonds form.
(The bond energies in are: ; ; ; ; ).
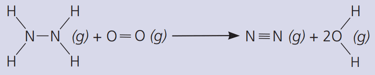

Important Questions on Energy Changes, and Reversible Reactions
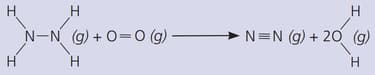
Calculate the energy change in the reaction.
(The bond energies in are: ; ; ; ; ).
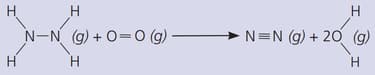
Is the reaction exothermic, or endothermic?
(The bond energies in are: ; ; ; ; )
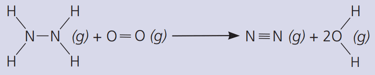
Where is energy transferred from, and to?
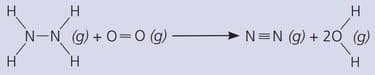
Comment on the suitability of hydrazine as a fuel.
(The bond energies in are: ; ; ; ; )
Hydrogen and bromine react reversibly:
undefined
Add more hydrogen will favour the formation of more hydrogen bromide?
Hydrogen and bromine react reversibly:
Remove bromine will favour the formation of more hydrogen bromide?
Hydrogen and bromine react reversibly:
undefined
Remove the hydrogen bromide as it forms will favour the formation of more hydrogen bromide?
Hydrogen and bromine react reversibly:
Explain why increasing the pressure will have no effect on the amount of product formed.
