MEDIUM
Earn 100
The gas in a vessel is subjected to a pressure of 20 atmosphere at a temperature . The pressure of the gas in the vessel after one half of the gas is released from the vessel and the temperature of the remainder is raised by , is -
(a)
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on Kinetic Theory
MEDIUM
An ideal gas undergoes a circular cycle centred at , as shown in the diagram.
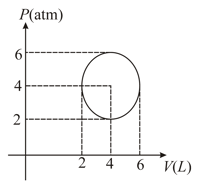
The maximum temperature attained in this process is close to
HARD
A long cylindrical pipe of radius is closed at its upper end and has an airtight piston of negligible mass as shown. When mass is attached to the other end of piston, it moves down by a distance, before coming to equilibrium. Assuming air to be an ideal gas, (see figure) is close to , one atmospheric pressure is ),
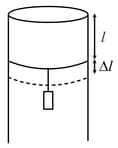
MEDIUM
EASY
EASY
EASY
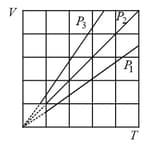
EASY
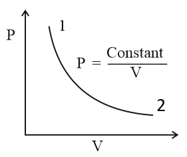
Out of the following which one correctly represents the diagram?
MEDIUM
MEDIUM
HARD
HARD
(Atmospheric pressure = of Hg)
EASY
MEDIUM
MEDIUM
MEDIUM
MEDIUM
MEDIUM
HARD
EASY
MEDIUM
( is universal gas constant and is the acceleration due to gravity)

