MEDIUM
NEET
IMPORTANT
Earn 100
The geometry of electron pairs around in is
(a)Octahedral
(b)Trigonal bipyramidal
(c)Square pyramidal
(d)Pentagonal planar
50% studentsanswered this correctly

Important Questions on Chemical Bonding and Molecular Structure
HARD
NEET
IMPORTANT
The correct order of increasing bond angles in the following triatomic species is:
EASY
NEET
IMPORTANT
In the hydrocarbon
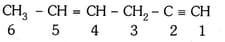
The state of hybridization of carbons are in the following sequence:-
MEDIUM
NEET
IMPORTANT
The is a planar molecule whereas is pyramidal because
EASY
NEET
IMPORTANT
Which compound has planar structure?
EASY
NEET
IMPORTANT
Which of the following pairs are isostructural?
MEDIUM
NEET
IMPORTANT
In which of the following bond angle is maximum?
MEDIUM
NEET
IMPORTANT
In molecule, the lone pairs occupy equatorial position to minimize:
HARD
NEET
IMPORTANT
Which of the following species has a linear shape?
