HARD
Earn 100
The graph below shows the observed standard electrode potential of some transition elements.
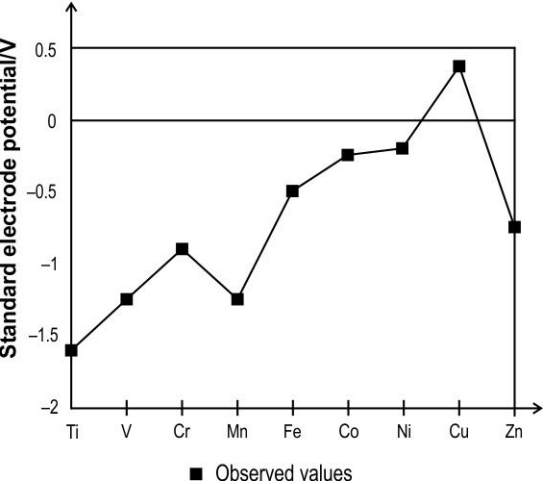
Which of the following reactions can be predicted based on the graph above?

(a)
(b)
(c)
(d)
25% studentsanswered this correctly
Important Questions on The d- and f-Block Elements
MEDIUM
(At.nos.
HARD
[Atomic number of Cr = 24 and Mn = 25]
EASY
MEDIUM
[Note: Ignore the pairing energy]
HARD
(Atomic Number of )
HARD
MEDIUM
MEDIUM
MEDIUM
EASY
MEDIUM
HARD
MEDIUM
EASY
Assertion: For hydrogenation reactions, the catalytic activity increases from Group to Group metals with maximum activity shown by Group elements.
Reason: The reactants are most strongly adsorbed on group elements.
HARD
Octahedral complexes with strong field ligands have very high magnetic moments
When , the d-electron configuration of in an octahedral complex is
Wavelength of light absorbed by is lower than that of
If the for an octahedral complex of is , the for its tetrahedral complex with the same ligand will be .
EASY
HARD
MEDIUM
HARD
MEDIUM

