The graph below shows the titration curve of of of hydrochloric acid with sodium hydroxide, of concentration. The indicator methyl orange was used to determine the equivalence point. Methyl orange has a pH range of .
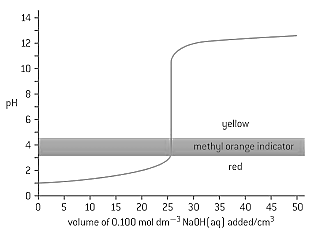
If the hydrochloric acid was replaced by ethanoic acid of the same volume and concentration, which property of the titration would remain the same?


Important Questions on Acids and Bases (AHL)
When the following aqueous solutions are arranged in order of increasing pH, which is the correct order?
I Ammonium chloride
II Ammonium ethanoate
III Sodium ethanoate
Predict and explain, using equations where appropriate, whether the following solution is acidic, alkaline, or neutral.
Predict and explain, using equations where appropriate, whether the following solution is acidic, alkaline, or neutral.
Predict and explain, using equations where appropriate, whether the following solutions are acidic, alkaline, or neutral.
A ammonia solution is placed in a flask and titrated with a hydrochloric acid solution.
Explain why the pH of the ammonia solution is less than .
