EASY
NEET
IMPORTANT
Earn 100
The graph between the concentration of the product and time of the reaction is of the type 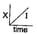 Hence, graph between and time will be of the type:
Hence, graph between and time will be of the type:
(a)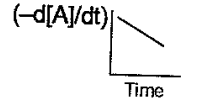
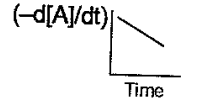
(b)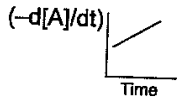

(c)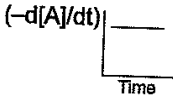
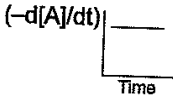
(d)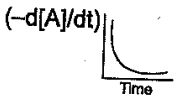
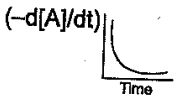
50% studentsanswered this correctly

Important Questions on Chemical Kinetics
MEDIUM
NEET
IMPORTANT
EASY
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
EASY
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
EASY
NEET
IMPORTANT
