The graph shows the variation with time of the activity of a radioactive sample,
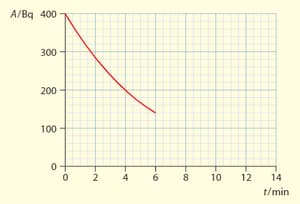
The sample contains a radioactive element that decays into a stable element . At , no atoms of are in the sample present
in the sample. Determine the time after which the ratio of atoms to atom is

Important Questions on Atomic, Nuclear and Particle Physics
The intensity of gamma rays of specific energy (monochromatic rays) decreases exponentially with the thickness of the absorbing material according to the equation
where = intensity at the face of the absorber and is a constant depending upon the material
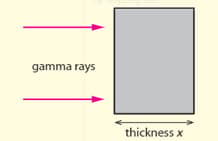
Discuss how the intensity and thickness should be plotted in order to allow an accurate determination of the constant .
A fission reaction involving uranium is:
Calculate the energy released.(in ) (Atomic masses: ; ; )
