EASY
JEE Main
IMPORTANT
Earn 100
The graph which depicts the results of Rutherford gold foil experiment with -particles is:
Scattering angle
Number of scattered -particles detected
(Plots are schematic and not to scale)
(a)
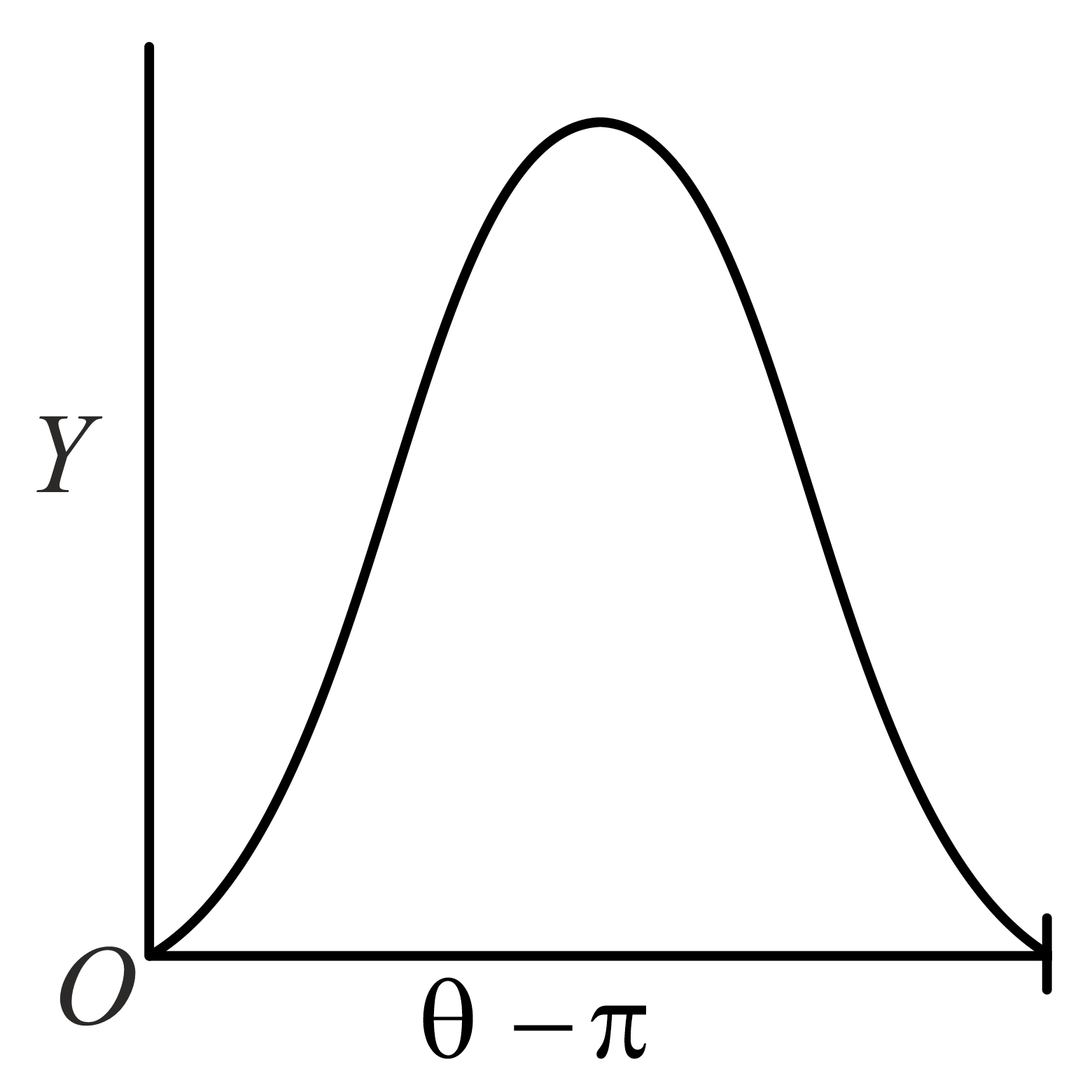
(b)
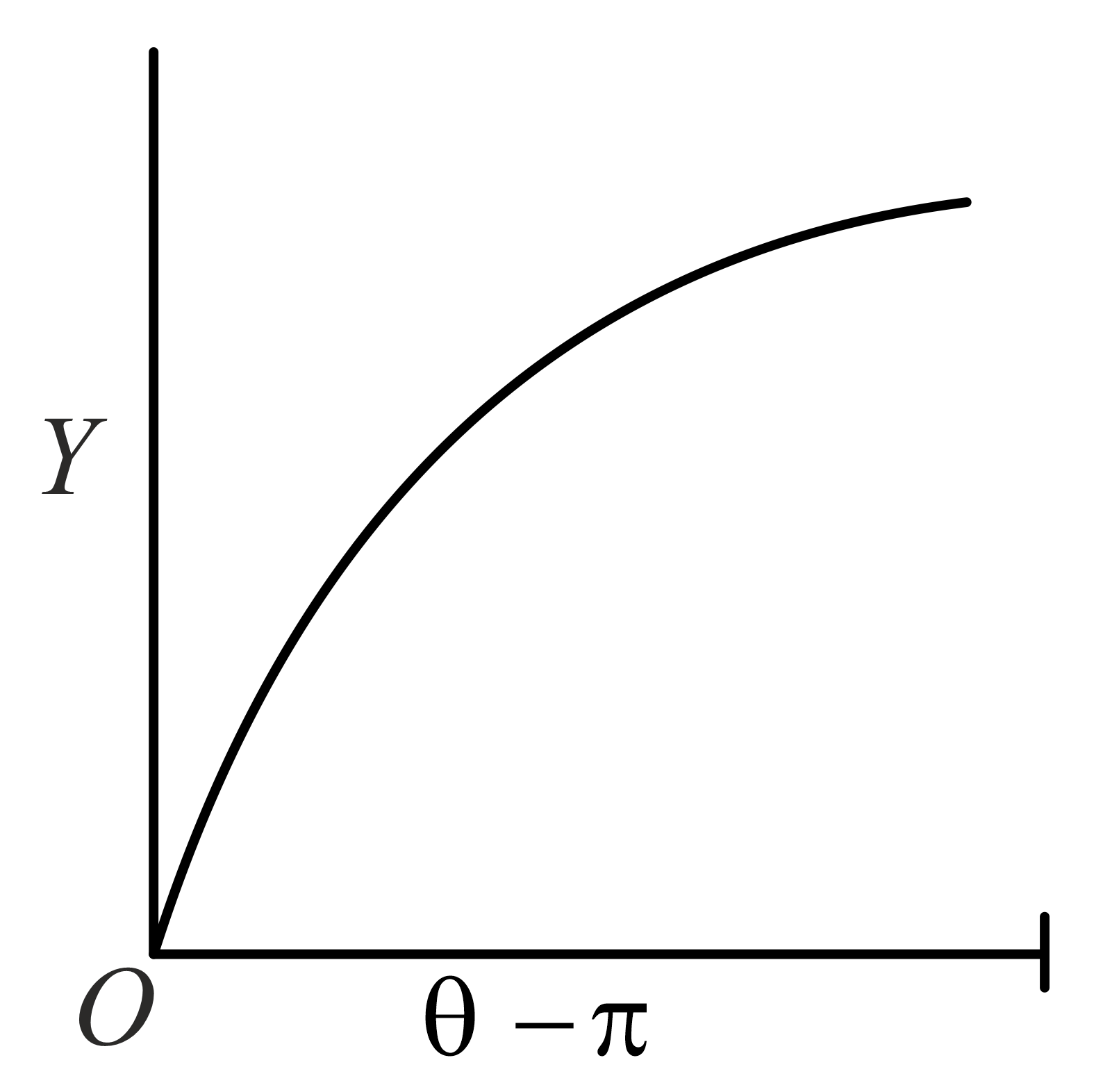
(c)
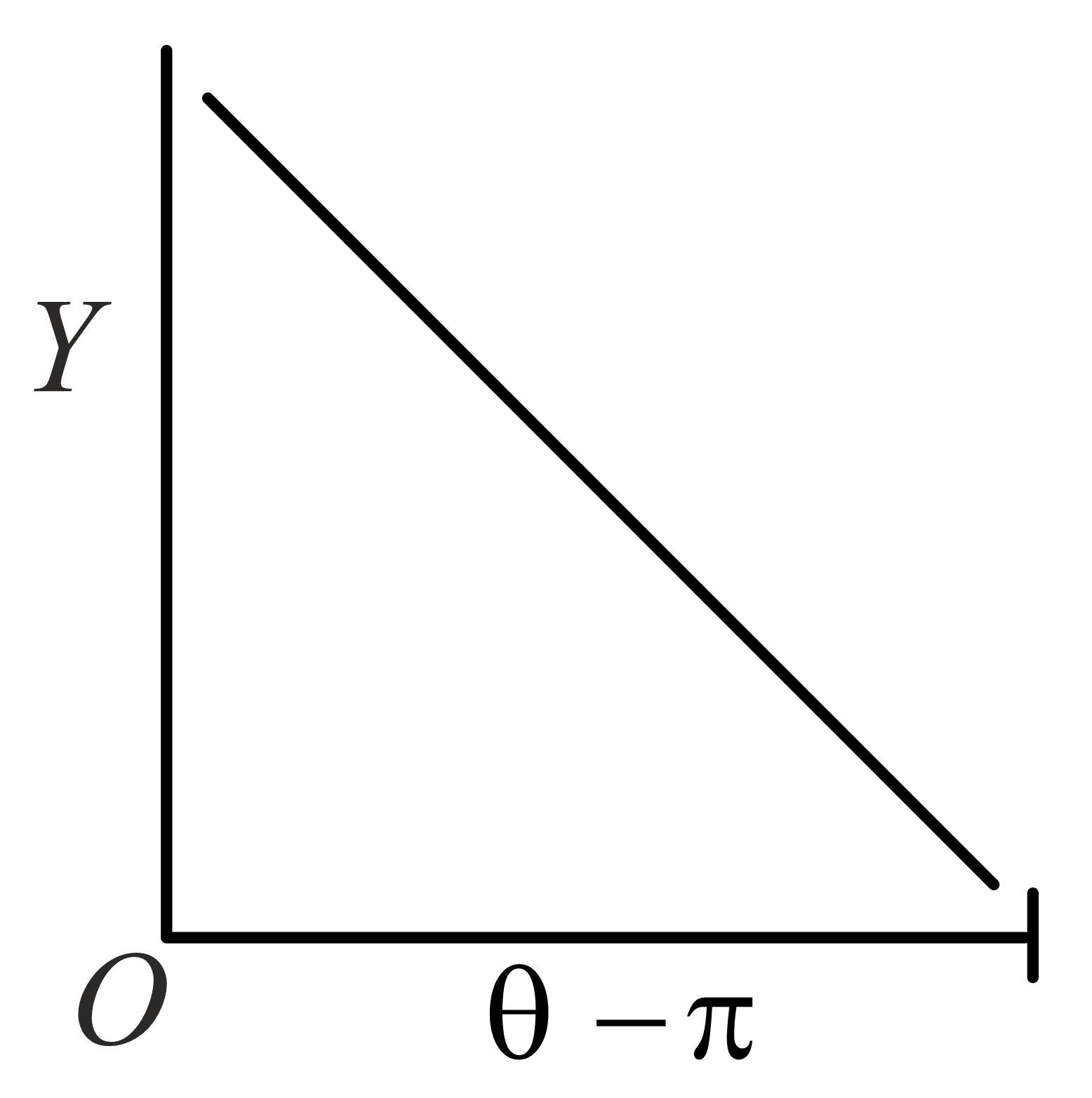
(d)
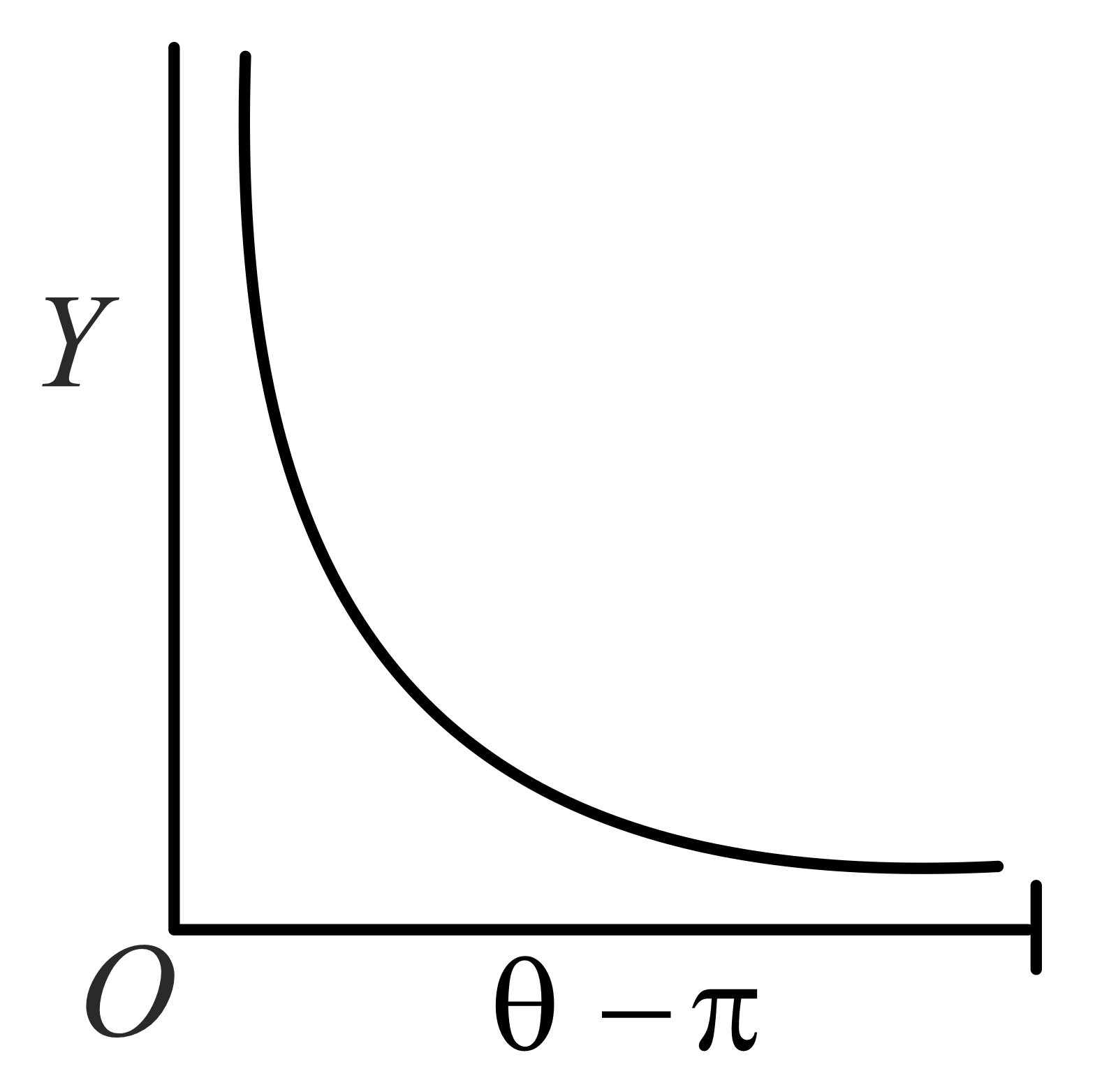
61.54% studentsanswered this correctly

Important Questions on Atoms and Nuclei
MEDIUM
JEE Main
IMPORTANT
The first member of the Balmer series of hydrogen atom has a wavelength of . The wavelength of the second member of the Balmer series (in nm) is_____________
MEDIUM
JEE Main
IMPORTANT
The energy required to ionise a hydrogen like ion in its ground state is Rydbergs. What is the wavelength of the radiation emitted when the electron in this ion jumps from the second excited state to the ground state?
MEDIUM
JEE Main
IMPORTANT
Taking the wavelength of first Balmer line in hydrogen spectrum to as , the wavelength of the Balmer line to will be :
MEDIUM
JEE Main
IMPORTANT
A ion is in its first excited state. Its ionization energy is:
EASY
JEE Main
IMPORTANT
A sample of radioactive material , that has an activity of has twice the number of nuclei as another sample of a different radioactive material which has an activity of . The correct choices for half-lives of and would then be, respectively:
MEDIUM
JEE Main
IMPORTANT
At a given instant, say two radioactive substance and have equal activities. The ratio of their activities after time itself decays with time as If the half-life of is the half-life of is:
EASY
JEE Main
IMPORTANT
Radiation coming from transitions to of hydrogen atoms fall on ions in and states. The possible transition of helium ions as they absorb energy from the radiation is:
EASY
JEE Main
IMPORTANT
The ratio of mass densities of nuclei of and is close to:
