MEDIUM
Earn 100
The group reagent for the test of alcohols is
(a)Ceric ammonium nitrate
(b)Schiff's reagent
(c)Molisch's reagent
(d)Bromine water
50% studentsanswered this correctly
Important Questions on Alcohols, Phenols and Ethers
HARD
What is the product "C" after following reactions -
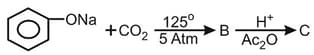
HARD
In the following reaction, the product(s) formed is/are:
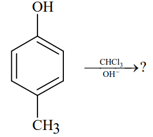
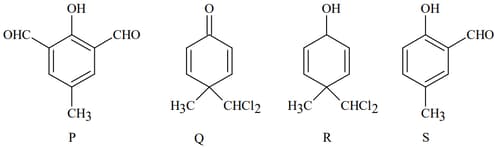
MEDIUM
Which one of the following is the most acidic compound?
HARD
Phenol on treatment with in the presence of NaOH followed by acidification produces compound X as the major product. X on treatment with O in the presence of catalytic amount of produces:
MEDIUM
The functional group which is formed when Phenol is made to react with Chloroform in the presence of dilute Sodium hydroxide
EASY
Which one of the following substituents at para-position is most effective in stabilizing the phenoxide
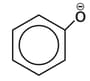 ion ?
ion ?HARD
The major product(s) of the following reaction is/are:
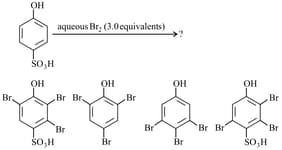
MEDIUM
The organic compound that gives following qualitative analysis is:
| Test | Inference | |
|---|---|---|
| Insoluble | ||
| Soluble | ||
| Decolourization |
MEDIUM
Arrange the following compounds in order of decreasing acidity :
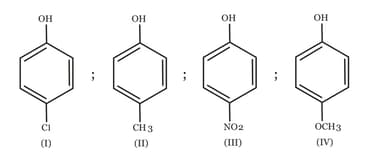
HARD
The major product of the following reaction is:
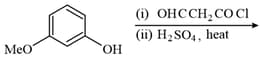
HARD
Phenol reacts with methyl chloroformate in the presence of to form product reacts with to form product are respectively
HARD
The increasing order of the values of the following compounds is:
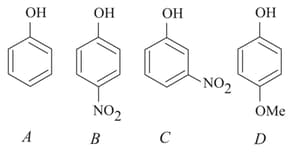
HARD
The reactivity of compound Z with different halogens under appropriate conditions is given below:
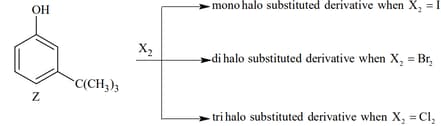
The observed pattern of electrophilic substitution can be explained by
MEDIUM
Which of the following compounds will not be soluble in sodium bicarbonate?
HARD
The major products of the following reaction are:
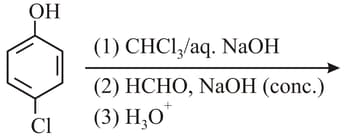
MEDIUM
Which of the following will not be soluble in sodium hydrogen carbonate?
MEDIUM
What will be the major product when m-cresol is reacted with propargyl bromide in presence of in acetone?
MEDIUM
The acid which contains both -OH and -COOH groups is
MEDIUM
The following reaction
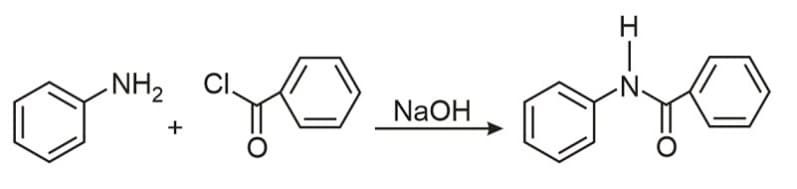
Is known by the name:
EASY
A compound is soluble in concentrated . It does not decolourise bromine in carbon tetrachloride but is oxidised by chromic anhydride in aqueous sulphuric acid within two seconds, turning orange solution to blue, green and then, opaque. The original compound is

