HARD
Earn 100
The half-life period of a first order reaction at is minutes. The time (in min.) required for completion of the reaction at the same temperature is.
(a)
(b)
(c)
(d)
(e)
50% studentsanswered this correctly
Important Questions on Chemical Kinetics
HARD
The decomposition reaction is started in a closed cylinder under the isothermal isochoric condition at an initial pressure of . After , the pressure inside the cylinder is found to be . If the rate constant of the reaction is , assuming ideal gas behaviour, the value of is(nearest integer)
MEDIUM
MEDIUM
MEDIUM
EASY
MEDIUM
MEDIUM
HARD
Which of the following plots is(are) correct for the given reaction?
( is the initial concentration of )
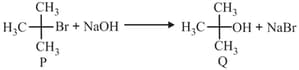
EASY
EASY
MEDIUM
(Take: )
HARD
(Assume that all these gases behave as ideal gases)
MEDIUM
HARD
MEDIUM
MEDIUM
MEDIUM
HARD
EASY
MEDIUM

