MEDIUM
JEE Main
IMPORTANT
Earn 100
The heat of combustion of ethanol into carbon dioxide and water is Kcal at constant pressure. The heat evolved (in cal) at constant volume at (if all gases behave ideally) is ________
50% studentsanswered this correctly

Important Questions on Thermodynamics
MEDIUM
JEE Main
IMPORTANT
The internal energy change (in ) when of water undergoes complete evaporation at is ..............
(Given : for water at , )
MEDIUM
JEE Main
IMPORTANT
For which of the following processes, is negative?
EASY
JEE Main
IMPORTANT
For which of the following reactions, is equal to ?
HARD
JEE Main
IMPORTANT
An ideal gas undergoes a cyclic process as shown in the figure
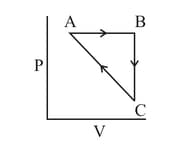
Heat absorbed by the system during process is
MEDIUM
JEE Main
IMPORTANT
Given:
Based on the above thermochemical equations, find out which one of the following algebraic relationships is correct?
MEDIUM
JEE Main
IMPORTANT
The combination of plots which does not represent isothermal expansion of an ideal gas is




MEDIUM
JEE Main
IMPORTANT
For a diatomic ideal gas in a closed system, which of the following plots does not correctly describe the relation between various thermodynamic quantities?
EASY
JEE Main
IMPORTANT
An ideal gas is allowed to expand from to against a constant external pressure of bar. The work done in is:
