HARD
Chemistry
IMPORTANT
Earn 100
The heat of formation of at from the following data will be:
The mean heat capacities over this temperature range are:
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Thermodynamics
HARD
Chemistry
IMPORTANT
Calculate the average bond energy in with the help of the following data.
HARD
Chemistry
IMPORTANT
The average bond energy is , first I.E. of is , electron affinity of is and bond dissociation energy of is . Then, the enthalpy change for the reaction
will be
HARD
Chemistry
IMPORTANT
EASY
Chemistry
IMPORTANT
MEDIUM
Chemistry
IMPORTANT
EASY
Chemistry
IMPORTANT
MEDIUM
Chemistry
IMPORTANT
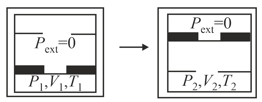
MEDIUM
Chemistry
IMPORTANT
