EASY
NEET
IMPORTANT
Earn 100
The hybridisation state of central atom in is
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Chemical Bonding and Molecular Structure
EASY
NEET
IMPORTANT
The shape of molecule is
EASY
NEET
IMPORTANT
Intramolecular -bonding is shown by
(i) 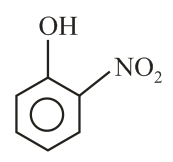
(ii) 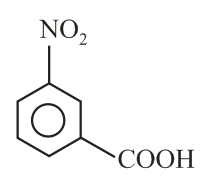
(iii) 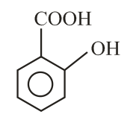
EASY
NEET
IMPORTANT
The compound possessing maximum covalent character among the following is :
EASY
NEET
IMPORTANT
According to octet rule the compound which contain ionic, covalent and coordinate bonds
EASY
NEET
IMPORTANT
Which of the following molecule is having least ionic character?
EASY
NEET
IMPORTANT
In ion, the effective charge on each oxygen atom and bond order respectively are
EASY
NEET
IMPORTANT
A certain diatomic molecule, has dipole moment and the internuclear distance is . The percentage of electronic charge existing on more electronegative atom is
EASY
NEET
IMPORTANT
Dipole moment of 
