EASY
JEE Main
IMPORTANT
Earn 100
The hybridisations of the atomic orbitals of nitrogen in and respectively are.
(a) and
(b) and
(c) and
(d) and
62.5% studentsanswered this correctly

Important Questions on Chemical Bonding and Molecular Structure
MEDIUM
JEE Main
IMPORTANT
According to the valence bond theory the hybridization of central metal atom is for which one of the following compounds?
EASY
JEE Main
IMPORTANT
The number of lone pairs of electrons on the central atom in is
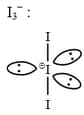
HARD
JEE Main
IMPORTANT
The shape/structure of and , respectively are :
MEDIUM
JEE Main
IMPORTANT
The molecular geometry of is octahedral. What is the geometry of (including lone pair(s) of electrons, if (any)?
MEDIUM
JEE Main
IMPORTANT
If molecule is a polar molecule, a possible geometry of is :
MEDIUM
JEE Main
IMPORTANT
Which one of the following molecules is paramagnetic?
MEDIUM
JEE Main
IMPORTANT
Amongst LiCl, RbCl, BeCl2 and MgCl2 the compounds with the greatest and the least ionic character, respectively are :
EASY
JEE Main
IMPORTANT
Which of the following conversions involves change in both shape and hybridisation?
