HARD
Earn 100
The hydrolysis reaction that takes place at the slowest rate, among the following is
(a)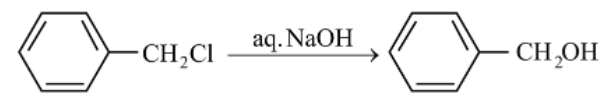
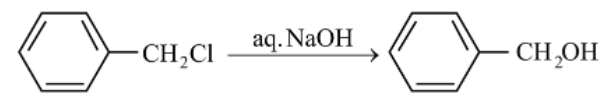
(b)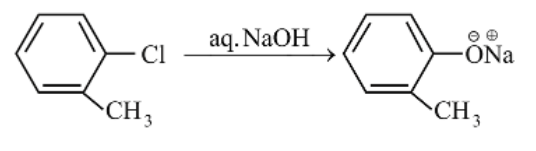

(c)
(d)
69.23% studentsanswered this correctly
Important Questions on Organic Compounds Containing Halogens
MEDIUM
MEDIUM
EASY
MEDIUM
MEDIUM
MEDIUM
Identify the relation among the rates of following nucleophilic substitution reactions?
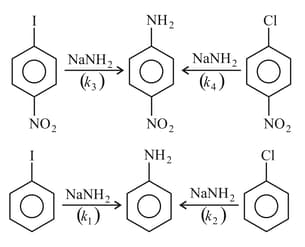
MEDIUM
EASY
The rates of reaction of with:
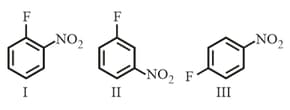
follow the order:-
EASY
EASY
EASY
MEDIUM
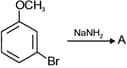
EASY
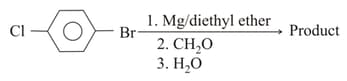
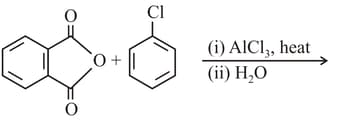
The product in the above reaction is
EASY
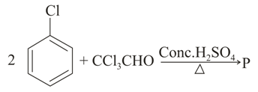
MEDIUM
EASY
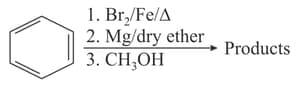
EASY
The correct order of the following compounds showing increasing tendency towards nucleophilic substitution reaction is:
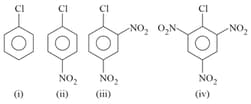
MEDIUM
MEDIUM
The major product of the following reaction is
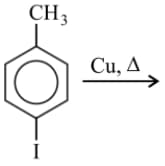
EASY

