MEDIUM
JEE Main/Advance
IMPORTANT
Earn 100
The hyperconjugative stabilities of tert-butyl cation and 2-butene, respectively, due to
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Organic Chemistry- Some Basic Principles and Techniques
EASY
JEE Main/Advance
IMPORTANT
In the following benzyl/allyl system
and 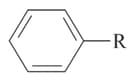 ( is alkyl group)
( is alkyl group)
Then decreasing order of inductive effect is:
MEDIUM
JEE Main/Advance
IMPORTANT
The non aromatic compound among the following is:
MEDIUM
JEE Main/Advance
IMPORTANT
Compound of molecular formula exists in keto form and predominantly in enolic form . On oxidation with gives -Chlorobenzoic acid. Identify and
MEDIUM
JEE Main/Advance
IMPORTANT
What is the acidity order of and
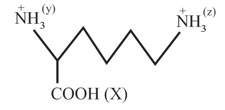
EASY
JEE Main/Advance
IMPORTANT
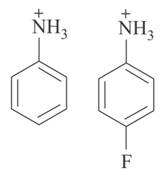
Which one of the following two compounds is the stronger acid? Explain why?
EASY
JEE Main/Advance
IMPORTANT
The correct stability order for the following species is:
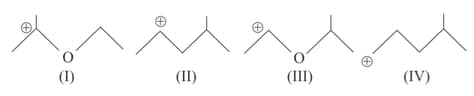
MEDIUM
JEE Main/Advance
IMPORTANT
The correct statements(s) concerning the structures and is (are):
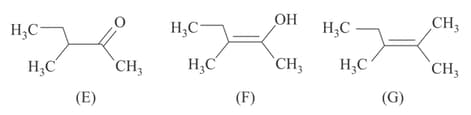
MEDIUM
JEE Main/Advance
IMPORTANT
The correct acidity order of the following is: