The image below shows the boiling point of first seven straight chain primary alcohols and first seven straight chain primary alkanes.
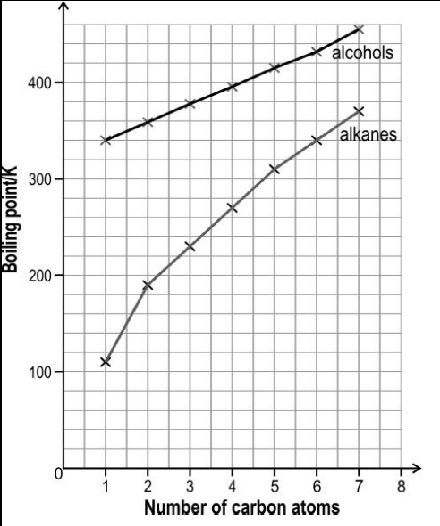
The boiling point of both the series increase monotonically with increasing size of the molecules. However, the slope of increment is different for both the series. Observe the above graph and answer the following question:
How will the boiling point graph for straight chain primary amines fare as compared to alcohols and alkanes?

Important Questions on Alcohols, Phenols and Ethers
Give the reasons :
Boiling points of alcohols are higher than ethers.
Given below are two statements : One is labelled as and the other is labelled as .
Butan––ol has higher boiling point than ethoxyethane.
Extensive hydrogen bonding leads to stronger association of molecules.
In the light of the above statements, choose the correct answer from the options given below :
Arrange the following alcohols in order of their increasing boiling points.
Pentan--ol Butan--ol Butan-ol Propane--ol Ethanol
I II III IV V

