MEDIUM
JEE Main
IMPORTANT
Earn 100
The incorrect geometry is represented by:
(a) - trigonal planar
(b) - trigonal planar
(c) - trigonal bipyramidal
(d) - bent
50% studentsanswered this correctly

Important Questions on Chemical Bonding and Molecular Structure
EASY
JEE Main
IMPORTANT
In the molecular orbital diagram for the molecular ion , the number of electrons in the molecular orbital is
MEDIUM
JEE Main
IMPORTANT
Identify the pair in which the shape of the species is T-shaped and square-pyramidal, respectively,
MEDIUM
JEE Main
IMPORTANT
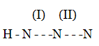
The above compound represents hydrogen azide, the bond orders of bonds and are:
MEDIUM
JEE Main
IMPORTANT
In graphite and diamond, the percentage of p-characters of the hybrid orbitals in hybridization respectively, are
MEDIUM
JEE Main
IMPORTANT
The decreasing order of bond angles in and is:
EASY
JEE Main
IMPORTANT
Among the following four aromatic compounds, which one will have the lowest melting point?
MEDIUM
JEE Main
IMPORTANT
The correct statement among the following is:
HARD
JEE Main
IMPORTANT
Molecule AB has a bond length of and a dipole moment of 0.38 D. The fractional charge on each atom(absolute magnitude) is:
