EASY
Earn 100
The incorrect statement for kinetic molecular theory of gases is
(a)Particles of a gas are always in random motion
(b)There exists forces of attraction between the particles of a gas at ordinary temperature and pressure
(c)Collision of gas molecules are perfectly elastic
(d)Different particles in the gas may have different speeds
50% studentsanswered this correctly
Important Questions on States of Matter
EASY
MEDIUM
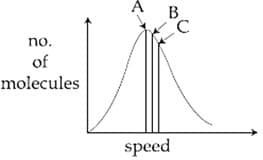
Root mean square speed most proable speed Average speed
EASY
MEDIUM
EASY
MEDIUM
HARD
If the distribution of molecular speeds of a gas is as per the figure shown below, then the ratio of the most probable, the average, and the root mean square speeds, respectively, is
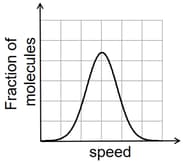
MEDIUM
MEDIUM
EASY
MEDIUM
MEDIUM
EASY
MEDIUM
EASY
EASY
EASY
MEDIUM
EASY
EASY

