EASY
JEE Main
IMPORTANT
Earn 100
The interaction energy of London forces between two particles is proportional to , where is the distance between the particles. The value of is :
(a)
(b)
(c)
(d)
70% studentsanswered this correctly

Important Questions on States of Matter: Gases and Liquids
EASY
JEE Main
IMPORTANT
An LPG cylinder contains gas at a pressure of at . The cylinder can withstand the pressure of . The room in which the cylinder is kept catches fire. The minimum temperature at which the bursting of cylinder will take place is __________ . (Nearest integer)
EASY
JEE Main
IMPORTANT
A home owner uses of methane gas, (assume is an ideal gas) in a year to heat his home. Under the pressure of atm and , mass of gas used is . The value of is ______. (Nearest integer)
(Given )
MEDIUM
JEE Main
IMPORTANT
A car tyre is filled with nitrogen gas at psi at . It will burst if pressure exceeds psi. The temperature in at which the car tyre will burst is (Rounded-off to the nearest integer)
EASY
JEE Main
IMPORTANT
The volume occupied by of acetylene gas at and pressure is ____________ (Rounded off to the nearest integer)
[Given ]
MEDIUM
JEE Main
IMPORTANT
Match the type of interaction in column with the distance dependence of their interaction energy in column
| A | B |
| (i) ion - ion | (a) |
| (ii) Dipole - dipole | (b) |
| (iii) London dispersion | (c) |
| (d) |
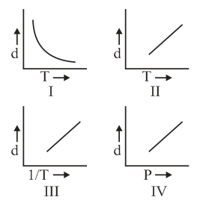
HARD
JEE Main
IMPORTANT
Which one of the following graphs is not correct for ideal gas?
Density, Pressure, Temperature
EASY
JEE Main
IMPORTANT
Sulphur dioxide and oxygen were allowed to diffuse through a porous partition. of diffuses through the porous partition in seconds. The volume of in which diffuses under the similar condition in seconds will be (atomic mass of sulphur);
MEDIUM
JEE Main
IMPORTANT
Assuming ideal gas behavior, the ratio of density of ammonia to that of hydrogen chloride at same temperature and pressure is: (Atomic weight of Cl is 35.5 u)
