The intermolecular attraction that is dependent on the inverse cube of the distance between the molecules is
Important Questions on States of Matter
In which of the following solid substance dispersion forces exist?
[ is the distance between the polar molecules]
Three types of potential energy due to attractive interaction between two species and are represented by the curves and in the figure below.
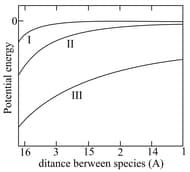
Consider the dominating interaction between non-rotating species for , and . The correct assignment of the ... interactions
to the types , and is:
Match the type of interaction in column with the distance dependence of their interaction energy in column
| A | B |
| (i) ion - ion | (a) |
| (ii) Dipole - dipole | (b) |
| (iii) London dispersion | (c) |
| (d) |
Intermolecular forces are forces of attraction and repulsion between interacting particles that will include :
A. dipole-dipole forces.
B. dipole-induced dipole forces.
C. hydrogen bonding.
D. covalent bonding.
E. dispersion forces.
Choose the most appropriate answer from the options given below:
Increasing order of boiling points in the following compounds is:

