MEDIUM
9th CBSE
IMPORTANT
Earn 100
The ion of an element has positive charges. Mass number of the atom is and the number of neutrons is . What is the number of electrons in the ion?
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Structure Of The Atom
HARD
9th CBSE
IMPORTANT
MEDIUM
9th CBSE
IMPORTANT
MEDIUM
9th CBSE
IMPORTANT
EASY
9th CBSE
IMPORTANT
MEDIUM
9th CBSE
IMPORTANT
MEDIUM
9th CBSE
IMPORTANT
MEDIUM
9th CBSE
IMPORTANT
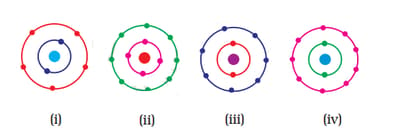
Which of the following figures do not represent Bohr's model of an atom correctly?
MEDIUM
9th CBSE
IMPORTANT
