MEDIUM
Earn 100
The ionic product of water ____ if a few drops of acid or base are added to it.
(a)increases
(b)decreases
(c)remains the same
(d)cannot predict
50% studentsanswered this correctly
Important Questions on Ionic Equilibria
EASY
MEDIUM
EASY
Which of the following equations give ionic product of water?
(i)
(ii)
(iii)
(iv)
HARD
HARD
Assertion: The of water increases with increase in temperature.
Reason: The dissociation of water into and is an exothermic reaction.
MEDIUM
[Kw of H2O = 10-12 M2 at 90oC]
MEDIUM
EASY
At , pure water has . What is the value of at ?
HARD
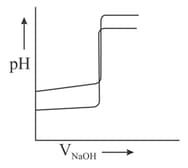
Which is/are correct statements?
(i) In any strong acid solution, the concentration of will be zero.
(ii) If of a reaction is positive, then the reaction will not proceed at all, in the directions of reactants and products.
(iii) Titration curves are drawn for (about the figure shown).
(a) with and
(b) with on the same graph paper they look like:
MEDIUM
Which of the following is incorrect for pure water at the given temperature?
(At the given temperature for water is )
MEDIUM
EASY
, is given as
HARD
EASY
EASY
EASY
EASY
EASY
EASY

