MEDIUM
JEE Main
IMPORTANT
Earn 100
The isomer(s) of that has/ have a angle of is/are:
34.48% studentsanswered this correctly
Important Questions on Coordination Compounds
HARD
JEE Main
IMPORTANT
Complex Of composition Has a spin only magnetic moment of It reacts with And shows geometrical isomerism. The IUPAC nomenclature of Is:
EASY
JEE Main
IMPORTANT
Homoleptic octahedral complexes of a metal ion with three monodentate ligands absorb wavelengths in the region of green, blue and red respectively. The increasing order of the ligand strength is:
MEDIUM
JEE Main
IMPORTANT
The complex that has highest crystal field splitting energy , is:
MEDIUM
JEE Main
IMPORTANT
Two complexes and are violet and yellow coloured, respectively, The incorrect statement regarding them is:
MEDIUM
JEE Main
IMPORTANT
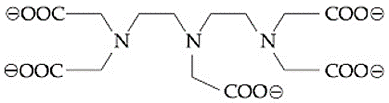
The maximum possible denticities of a ligand given below towards a common transition and inner-transition metal ion, respectively, are:
MEDIUM
JEE Main
IMPORTANT
The correct statements among I to III are:
(I) Valence bond theory cannot explain the color exhibited by transition metal complexes.
(II) Valence bond theory can predict quantitatively the magnetic properties of transition metal complexes.
(III) Valence bond theory cannot distinguish ligands as weak and strong field ones.
EASY
JEE Main
IMPORTANT
The degenerate orbitals of are
MEDIUM
JEE Main
IMPORTANT
The one that will show optical activity is
(ethanediamine)
