MEDIUM
Earn 100
The isothermal compressibility factor '' For an ideal gas at Pressure is
(a)
(b)2
(c)5
(d)3
50% studentsanswered this correctly
Important Questions on States of Matter
MEDIUM
EASY
MEDIUM
HARD
HARD
[Given : " a " and " b " are standard parameters for van der Waals' gas]
MEDIUM
EASY
MEDIUM
(R = 8.314 J/mol K) (ln7.5 = 2.01)
MEDIUM
EASY
MEDIUM
The combination of plots which does not represent isothermal expansion of an ideal gas is
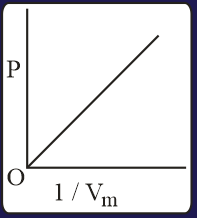
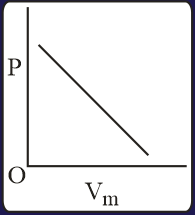
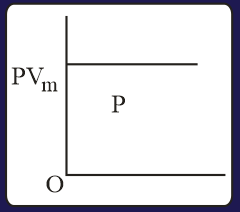
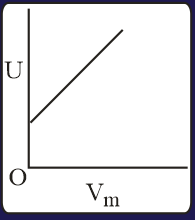
HARD
HARD
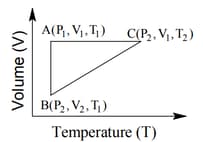
The correct option(s) is (are)
EASY
(Latent heat of ice is and )
HARD
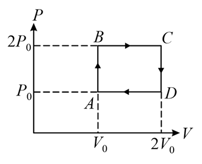
The above diagram represents the thermodynamic cycle of an engine, operating with an ideal mono-atomic gas. The amount of heat, extracted from the source in a single cycle, is:
EASY
MEDIUM
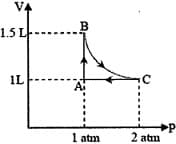
HARD
The isotherms of a gas are shown below :
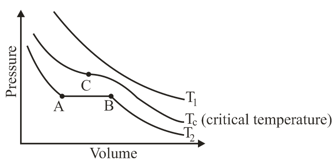
Among the following,
(i) At , the gas cannot be liquefied
(ii) At point , liquid starts to appear at
(ii) is the highest temperature at which the gas can be liquefied
(iv) At point , a small increase in pressure condenses the whole system to a liquid
The correct statements are :
MEDIUM
EASY


