HARD
Earn 100
The isotherms of a gas are shown below :
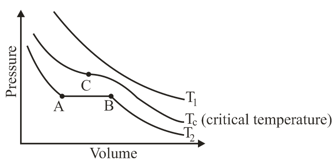
Among the following,
(i) At , the gas cannot be liquefied
(ii) At point , liquid starts to appear at
(ii) is the highest temperature at which the gas can be liquefied
(iv) At point , a small increase in pressure condenses the whole system to a liquid
The correct statements are :

(a)only (i) and (ii)
(b)only (i), (iii) and (iv)
(c)only (ii), (iii) and (iv)
(d)(i), (ii), (iii) and (iv)
50% studentsanswered this correctly
Important Questions on States of Matter
EASY
(Latent heat of ice is and )
EASY

MEDIUM
MEDIUM
The combination of plots which does not represent isothermal expansion of an ideal gas is




MEDIUM
HARD
MEDIUM
MEDIUM
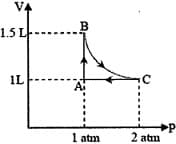
EASY
EASY
HARD

The correct option(s) is (are)
MEDIUM
HARD

The above diagram represents the thermodynamic cycle of an engine, operating with an ideal mono-atomic gas. The amount of heat, extracted from the source in a single cycle, is:
MEDIUM

MEDIUM
(R = 8.314 J/mol K) (ln7.5 = 2.01)
EASY
EASY
EASY
HARD
[Given : " a " and " b " are standard parameters for van der Waals' gas]
HARD

