The kinetic data for the given reaction is provided in the following table for three experiments at
Ex. No.
[Initial rate ]
1
2
3
In another experiment starting with initial concentration of and respectively for and at Find the rate of reaction after minutes from start of experiment (in )?

Important Questions on Chemical Kinetics
In three different reactions, involving a single reactant in each case, a plot of the rate of the reaction on the -axis, versus the concentration of the reactant on the -axis, yields three different curves are shown below.
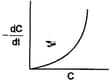
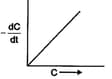
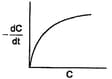
What are the possible orders of the reactions ?
Which integrated equation is correct for the following order reaction started with only in a closed rigid vessel?
initial pressure, total pressure at time .
The following data were obtained in an experiment on inversion of cane sugar; (a first order kinetics)
| Time | After a long time | ||
| Total angle of rotation (degree) |
The rate constant (in ) is
The decomposition of in chloroform was followed by measuring the volume of O2 gas evolved : ()() + . The maximum volume of gasobtained was. In 500 minutes, of were evolved. The first order rate constant for the disappearance of is :
