MEDIUM
12th West Bengal Board
IMPORTANT
Earn 100
The largest wavelength in the ultraviolet region of the hydrogen spectrum is . The smallest wavelength in the infrared region of hydrogen spectrum to the nearest
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Bohr Model, X-Ray Spectra, Wave-Particle Duality
EASY
12th West Bengal Board
IMPORTANT
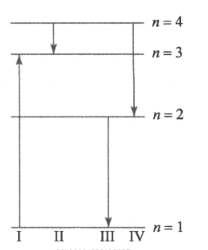
The diagram Figure shows the energy levels for an electron in a certain atom. Which transition shown represents the emission of a photon with the most energy.
EASY
12th West Bengal Board
IMPORTANT
Electrons in a certain energy level , can emit spectral lines. When they are in another energy level, , they can emit spectral lines. The orbital speed of the electrons in the orbit
EASY
12th West Bengal Board
IMPORTANT
Three photons coming from excited atomic hydrogen sample are observed, their energies are, and . These photons must come from
EASY
12th West Bengal Board
IMPORTANT
If the binding energy of the electron in a hydrogen atom is , the energy required to remove the electron from the first excited state of is
MEDIUM
12th West Bengal Board
IMPORTANT
What is the wavelength for doubly ionised lithium ion for the same transition? If is the wavelength of a hydrogen atom from the transition to .
MEDIUM
12th West Bengal Board
IMPORTANT
Which state of triply ionised beryllium has the same orbital radius as that of the ground state of hydrogen?
MEDIUM
12th West Bengal Board
IMPORTANT
The excitation energy of a hydrogen like ion in its first excitation state is . The energy needed to remove the electron from the ion in ground state is,
MEDIUM
12th West Bengal Board
IMPORTANT
Wavelength of light emitted from second orbit to first orbit in a hydrogen atom is
