MEDIUM
Earn 100
The law of triads is not applicable on:
(a)
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on The Periodic Table
EASY
EASY
EASY
MEDIUM
HARD
MEDIUM
MEDIUM
Chlorine, Bromine, and Iodine form a Dobereiner's Triad. If the approximate atomic mass of Chlorine is 35 and that of Iodine is 127:
Find the atomic mass of Bromine.
MEDIUM
In Dobereiner's triads , the atomic masses of lithium and potassium are and respectively, then what will be the atomic mass of sodium.
MEDIUM
HARD
(ii) Name the scientist who framed the above law.
(iii) Two elements A and B obey the law of octaves. How many elements are in between A and B?
MEDIUM
HARD
(ii) What were the reasons for rejecting the law of octaves?
HARD
Give one example of such a set of elements.
MEDIUM
HARD
(ii) What were the reasons for rejecting this classification?
MEDIUM
EASY
HARD
Rearrange the columns and to match with the column .
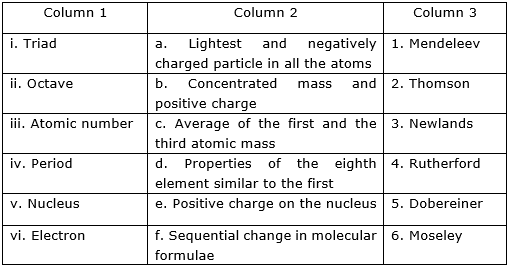
MEDIUM
HARD
Elements have been arranged in the following sequence on the basis of their increasing atomic masses.
(a) Pick two sets of elements that have similar properties.
(b) The given sequence represents what law of classification of elements?

