MEDIUM
KVPY Aptitude Test - Stream SA
IMPORTANT
Earn 100
The lowest stability of ethyl anion compared to methyl anion and the higher stability of ethyl radical compared to methyl radical, respectively, are due to:
(a) effect of the methyl group in ethyl anion and -orbital conjugation in ethyl radical.
(b) effect of the methyl group in ethyl anion and conjugation in ethyl radical.
(c) effect of the methyl group in both cases
(d) effect of the methyl group in ethyl anion and conjugation in ethyl radical.
50% studentsanswered this correctly

Important Questions on Electronic Effects & Applications
HARD
KVPY Aptitude Test - Stream SA
IMPORTANT
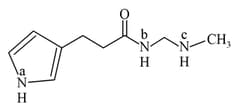
The most acidic proton and the strongest nucleophilic nitrogen in the following compound respectively, are: