MEDIUM
JEE Main
IMPORTANT
Earn 100
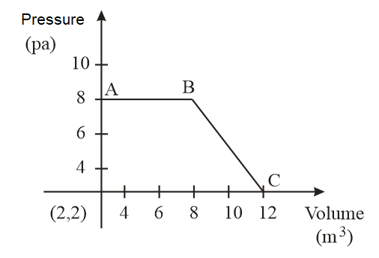
The magnitude of work done by a gas that undergoes a reversible expansion along the path shown in the figure is _________.

20% studentsanswered this correctly

Important Questions on Thermodynamics
MEDIUM
JEE Main
IMPORTANT
moles of an ideal gas at are allowed to undergo reversible compression till its temperature becomes If calculate and for the process.
MEDIUM
JEE Main
IMPORTANT
For silver, If the temperature of moles of silver is raised from pressure, the value of will be close to:
MEDIUM
JEE Main
IMPORTANT
Which one of the following equations does not correctly represent the first law of thermodynamics for the given processes involving an ideal gas? (Assume non- expansion work is zero)
MEDIUM
JEE Main
IMPORTANT
The combustion of benzene gives and Given that heat of combustion of benzene at constant volume is at the heat of combustion of benzene at constant pressure will be
EASY
JEE Main
IMPORTANT
The enthalpy change on freezing of of water at to ice at is:
(Given at
)
EASY
JEE Main
IMPORTANT
For a reaction, The correct statement for the reaction is
HARD
JEE Main
IMPORTANT
The standard heat of formation of ethane is , if the heat of combustion of ethane, hydrogen and graphite are and , respectively. The value of to the nearest integer is_____
MEDIUM
JEE Main
IMPORTANT
For the reaction;
at
Hence in is _____________.
