MEDIUM
JEE Main
IMPORTANT
Earn 100
The maximum possible denticities of a ligand given below towards a common transition and inner-transition metal ion, respectively, are:
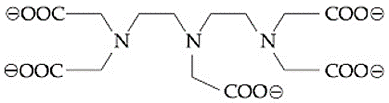
(a)8 and 6
(b)6 and 6
(c)8 and 8
(d)6 and 8
39.13% studentsanswered this correctly

Important Questions on Coordination Compounds
MEDIUM
JEE Main
IMPORTANT
The correct statements among I to III are:
(I) Valence bond theory cannot explain the color exhibited by transition metal complexes.
(II) Valence bond theory can predict quantitatively the magnetic properties of transition metal complexes.
(III) Valence bond theory cannot distinguish ligands as weak and strong field ones.
EASY
JEE Main
IMPORTANT
The degenerate orbitals of are
MEDIUM
JEE Main
IMPORTANT
The one that will show optical activity is
(ethanediamine)
EASY
JEE Main
IMPORTANT
displays
MEDIUM
JEE Main
IMPORTANT
Which one of the following complexes will consume more equivalents of aqueous solution of ?
MEDIUM
JEE Main
IMPORTANT
Identify the correct trend given below:
(Atomic No. = Ti : 22, Cr : 24 and Mo : 42)
MEDIUM
JEE Main
IMPORTANT
The correct statement about the magnetic properties of [Fe(CN)6]3- and [FeF6]3- is : (Z = 26).
MEDIUM
JEE Main
IMPORTANT
An octahedral complex of Co3+ is diamagnetic. The hybridisation involved in the formation of the complex is :
