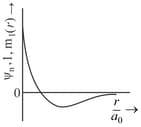
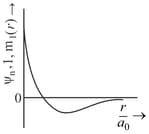
MEDIUM
Earn 100
The mean free path of a neutron in vacuum is closest to (Life time of neutron is about ) -
(a)
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on Structure of Atom
MEDIUM
MEDIUM
EASY
MEDIUM
The wavelength (in ) of a particle of mass moving with a velocity of is ______
MEDIUM
MEDIUM
EASY
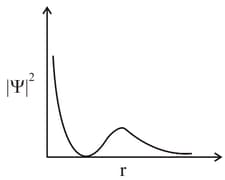
MEDIUM
HARD
| Column – 1 | Column – 2 | Column – 3 |
| (I) orbital | (i) |
(P) |
| (II) orbital | (ii) One radial node | (Q) Probability density at nucleus |
| (III) orbital | (iii) | (R) Probability density is maximum at nucleus |
| (IV) orbital | (iv) -plane is a nodal plane | (S) Energy needed to excite an electron from state to state is times the energy needed to excite electron from state to state. |
HARD
| Column 1 | Column 2 | Column 3 |
| (I) orbital | (i) |
(P) |
| (II) orbital | (ii) One radial node | (Q) Probability density at the nucleus . |
| (III) orbital | (iii) | (R) Probability density is maximum at the nucleus. |
| (IV) orbital | (iv) -plane is a nodal plane | (S) Energy needed to excite an electron from state to state is times the energy needed to excite are electron from state to state. |
EASY
MEDIUM
EASY
MEDIUM
HARD
The correct plot for orbital is :
MEDIUM
MEDIUM
MEDIUM
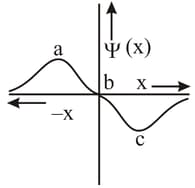
MEDIUM
[Planck's constant ]
MEDIUM