HARD
Earn 100
The mechanism of esterification in presence of acid catalyst is proposed as follows:
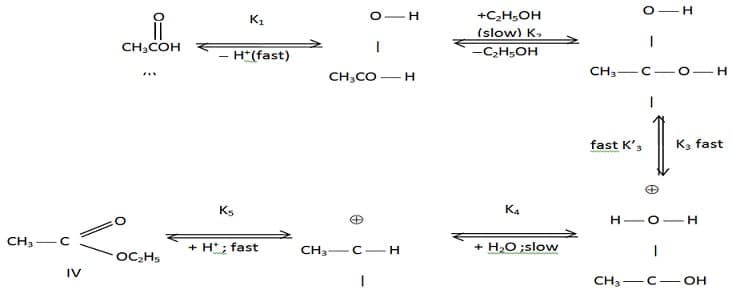
Which of the following potential energy vs reaction co-ordinate diagram is consistent with given mechanism?
(a)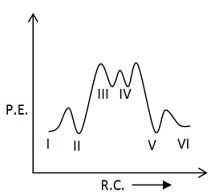

(b)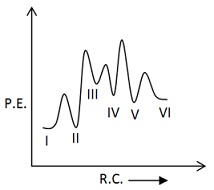

(c)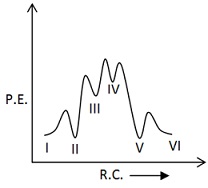

(d)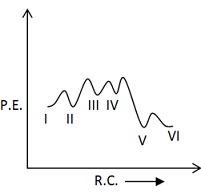

50% studentsanswered this correctly
Important Questions on Chemical Kinetics
EASY
HARD
EASY
MEDIUM
The reaction rate for the reaction
was measured as a function of concentrations of different species. It was observed that
where square brackets are used to denote molar concentrations. The equilibrium constant
(Nearest integer)
Value of (question is modified.)
EASY
MEDIUM
EASY
MEDIUM
The reaction takes place through the mechanism given below
The overall order of the reaction is _____.
EASY
MEDIUM
MEDIUM
in
is equal to __________ . (Integer answer)
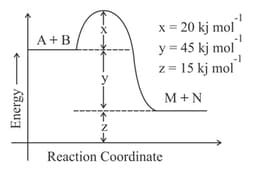
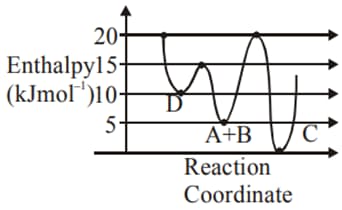
MEDIUM
Identify the incorrect statement.
MEDIUM
The energy of activation in is ________ . (Nearest integer)
[Given : ]
MEDIUM
The reaction is an elementary reaction.
For a certain quantity of reactants, if the volume of the reaction vessel is reduced by a factor of the rate of the reaction increases by a factor of _____ . (Round off to the Nearest Integer).
HARD
Consider the following reaction that goes from to in three steps as shown below:
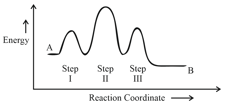
Choose the correct option
| Number of Intermediates |
Number of Activated Complexes |
Rate determining step |
|
EASY
EASY
(i)
(ii)
In the reaction,
HARD
HARD
It was found that the is decreased by in the presence of catalyst. If the rate remains unchanged, the activation energy for catalysed reaction is (Assume pre-exponential factor is same)
EASY

