HARD
8th Kerala Board
IMPORTANT
Earn 100
The metals like zinc, aluminium, iron and magnesium are added in different test tubes containing dilute hydrochloric acid. Answer the following questions based on your observations.
- Which of these metals reacted with the acid?
- Which of these metals reacted most vigorously?
- Is there any metal which did not react with the acid?
- Which gas is liberated during these reactions? How would you detect it?
- Is there any difference in the vigour of the reaction of the metals with acid?
Important Questions on Metals
HARD
8th Kerala Board
IMPORTANT
HARD
8th Kerala Board
IMPORTANT
MEDIUM
8th Kerala Board
IMPORTANT
HARD
8th Kerala Board
IMPORTANT
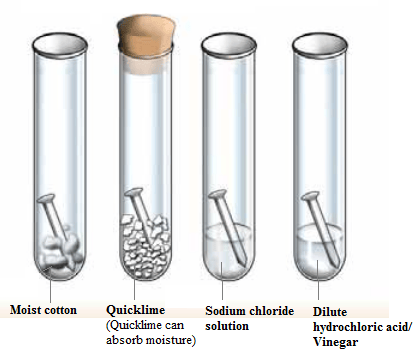
Analyse the given diagram. Recall the changes that occur to the iron nails if they are kept for one week and answer the following questions.
- Which test tubes had their nails rusted?
- Which test tubes had the maximum amount of rust in the iron nails kept in them?
- What are the factors which favour the corrosion of an iron article kept exposed to atmosphere?
- Which of the iron nails did not rust? What could be the reason?
HARD
8th Kerala Board
IMPORTANT
HARD
8th Kerala Board
IMPORTANT
HARD
8th Kerala Board
IMPORTANT
HARD
8th Kerala Board
IMPORTANT
Some metals are listed below. Complete the table by identifying the different uses and the properties which are responsible for the same.
| Metal | Use | Property |
| Gold |
|
|
| Copper |
|
|
| Aluminium |
|
|
| Zinc |
|
|
| Iron |
|
