The molality of a solution of di-bromine solution in (carbon tetrachloride) is '. . (Nearest integer)
[Given : molar mass of
atomic mass of
atomic mass of
density of dibromine
density of

Important Questions on Some Basic Concepts of Chemistry
of solution is titrated against solution. The following values were obtained in readings. and
Based on these readings, and convention of titrimetric estimation of concentration of solution is ___ .
(Round off to the Nearest integer)
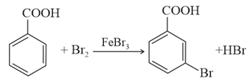
Consider the above reaction where of benzoic acid is used to get of m-bromo benzoic acid. The percentage yield of the product is ___ .
(Round off to the Nearest integer)
[Given : Atomic masses : ]
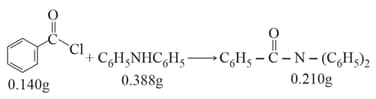
Consider the above reaction. The percentage yield of amide product is (Round off to the Nearest Integer). (Given : Atomic mass :
