MEDIUM
Earn 100
The molar conductivities of and are and respectively. The molar conductivity of would be _____
(a)
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on Electrochemistry
EASY
HARD
MEDIUM
MEDIUM
HARD
EASY
MEDIUM
HARD
A aqueous solution of has a conductance of when measured in a cell constant . The molar conductivity of this solution is _______ . (Round off to the Nearest Integer)
EASY
The variation of molar conductively with concentration of an electrolyte (X) in aqueous solution is shown in the given figure.
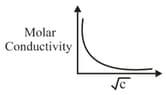
The electrolyte X is :
EASY
MEDIUM
EASY
: Conductivity always increases with decreases in the concentration of electrolyte.
: Molar conductivity always increases with decreases in the concentration of electrolyte.
The correct option among the following
MEDIUM
EASY
EASY
MEDIUM
Given below are two statements:
Statement I : The limiting molar conductivity of (strong electrolyte) is higher compared to that of $\mathrm{CH}_{3} \mathrm{COOH}$ (weak electrolyte).
Statement II : Molar conductivity decreases with decrease in concentration of electrolyte.
In the light of the above statements, choose the most appropriate answer from the options given below:
EASY
MEDIUM
MEDIUM
HARD

