EASY
Earn 100
The molar heat capacity of oxygen gas at STP is nearly 2.5R. As the temperature is increased, it gradually increases and approaches 3.5 R. The most appropriate reason for this behaviour is that at high temperature
(a)Oxygen does not behave as an ideal gas
(b)Oxygen molecules dissociate in atoms
(c)The molecules collide more frequently
(d)Molecular vibrations gradually become effective
50% studentsanswered this correctly
Important Questions on Thermodynamics
MEDIUM
HARD
MEDIUM
MEDIUM
What will be the molar specific heat at constant volume of an ideal gas consisting of rigid diatomic molecules?
EASY
EASY
HARD
MEDIUM
EASY
MEDIUM
MEDIUM
MEDIUM
HARD
EASY
EASY
EASY
MEDIUM
Two different metal bodies and of equal mass are heated at a uniform rate under similar conditions. The variation of temperature of the bodies is graphically represented as shown in the figure. The ratio of specific heat capacities is:
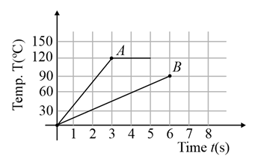
MEDIUM
[Given that
EASY
EASY
(Take gas constant )

