EASY
JEE Main
IMPORTANT
Earn 100
The molar specific heat of the process , for gas, at room temperature, is
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Thermodynamics
EASY
JEE Main
IMPORTANT
EASY
JEE Main
IMPORTANT
The first law of thermodynamics is a special case of,
EASY
JEE Main
IMPORTANT
EASY
JEE Main
IMPORTANT
A given quantity of gas is taken from the state to the state reversibly by two paths, directly and as shown in the figure below:
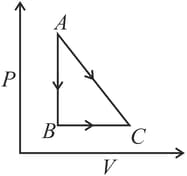
During the process , work done by the gas is and heat absorbed is . If during the process , the work done by the gas is , the heat absorbed is
MEDIUM
JEE Main
IMPORTANT
MEDIUM
JEE Main
IMPORTANT
EASY
JEE Main
IMPORTANT
EASY
JEE Main
IMPORTANT
