HARD
Earn 100
The molecular shapes of and are
(a)the same with and lone pairs of electrons on the central atoms, respectively.
(b)the same with and lone pair of electrons on each of the central atoms.
(c)different with and lone pairs of electrons on the central atoms, respectively.
(d)different with and lone pairs of electrons on the central atoms respectively.
50% studentsanswered this correctly
Important Questions on Chemical Bonding and Molecular Structure
EASY
MEDIUM
EASY
EASY
EASY
EASY
| Column I | Column II |
| a. | (i) T-shape |
| b. | (ii) Pentagonal bipyramidal |
| c. | (iii) Linear |
| d. | (iv) Square – pyramidal |
| (v) Tetrahedral |
MEDIUM
MEDIUM
EASY
EASY
(i)
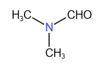 (ii)
(ii) 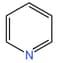
(iii) (iv)
MEDIUM
MEDIUM
EASY
EASY
HARD
EASY
EASY
MEDIUM
MEDIUM
EASY

