MEDIUM
Earn 100
The most polar compound among the following is:
(a)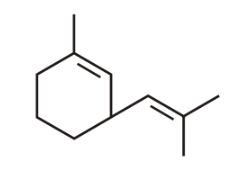

(b)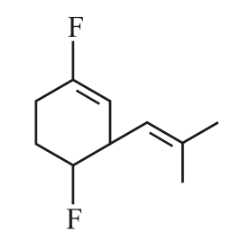

(c)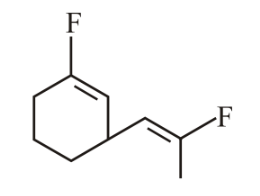

(d)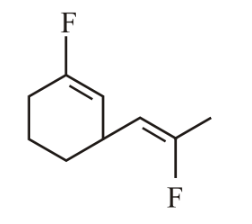

(e)Benzene
50% studentsanswered this correctly
Important Questions on Haloalkanes and Haloarenes(OoS)
HARD
MEDIUM
EASY
EASY
MEDIUM
MEDIUM
The major product of the following reaction is:
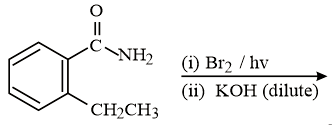
HARD
MEDIUM
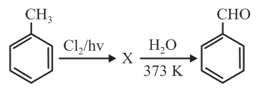
MEDIUM
EASY
HARD
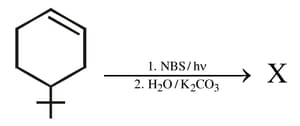
EASY
HARD
| Toulene | Condensation | |
| Acetophenone | Carboxylation | |
| Benzaldehyde | Substitution | |
| Phenol | Haloform |
EASY
EASY
HARD
Identify compound in the following reaction
HARD
In the following reaction, compound is obtained from compound via an ionic intermediate.
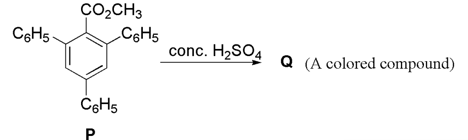
What is the degree of unsaturation of ?
MEDIUM
EASY
EASY

