EASY
Earn 100
The name of the ring structure complex formed between metal ion and polydentate ligand is ..........
(a)Polynuclear complex
(b)Chelate complex
(c)Simple complex
(d)None of these
50% studentsanswered this correctly
Important Questions on Coordination Compounds
EASY
EASY
EASY
is a monoanion having pyramidal geometry. Both and are neutral compounds. Choose the correct option(s).
EASY
MEDIUM
MEDIUM
EASY
EASY
EASY
EASY
The sum of coordination number and oxidation number of metal in the complex (Where en is ethylenediamine) is:
MEDIUM
MEDIUM
EASY
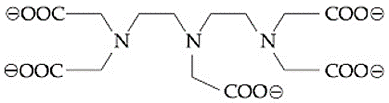
The total number of coordination sites in ethylenediaminetetraacetate ( ) is ...................
EASY
(en ethane-1, 2-diamine)
MEDIUM
HARD
MEDIUM
EASY
EASY
MEDIUM