EASY
JEE Main/Advance
IMPORTANT
Earn 100
The nature of -bonds in perchlorate ion is :-
(a)
(b)
(c)
(d)
100% studentsanswered this correctly

Important Questions on Chemical Bonding and Molecular Structure
HARD
JEE Main/Advance
IMPORTANT
and have the same crystal structure and approximately the same radii. If is the lattice energy of , the approximate lattice energy of is
MEDIUM
JEE Main/Advance
IMPORTANT
The ease of hydrolysis of trichlorides of group elements decreases in the order:-
MEDIUM
JEE Main/Advance
IMPORTANT
Which of the following solid sold have highest value of when heated in closed vessel:-
EASY
JEE Main/Advance
IMPORTANT
Type of bonds between calcium and carbon in are :-
MEDIUM
JEE Main/Advance
IMPORTANT
Ethanol has a higher boiling point than dimethyl ether though they have the same molecular weight. This is due to:
EASY
JEE Main/Advance
IMPORTANT
Write order of dipole moment of following compounds :-
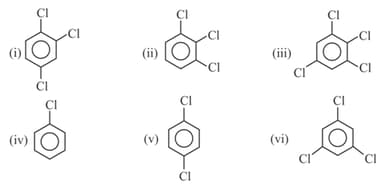
MEDIUM
JEE Main/Advance
IMPORTANT
The correct order of increasing bond angle is:
HARD
JEE Main/Advance
IMPORTANT
Out of given reaction which show change in hybridisation of central atom:-
