EASY
Earn 100
The normal boiling point of water is 373 K (at 760 mm). Vapour pressure of water at 298 K is 23 mm. If enthalpy of vaporisation is 40.656 kJ/mol, the boiling point of water at 23 mm atmospheric pressure will be-
(a)250 K
(b)51.6 K
(c)298 K
(d)12.5 K
50% studentsanswered this correctly
Important Questions on States of Matter
MEDIUM
MEDIUM
The combination of plots which does not represent isothermal expansion of an ideal gas is




HARD

The above diagram represents the thermodynamic cycle of an engine, operating with an ideal mono-atomic gas. The amount of heat, extracted from the source in a single cycle, is:
EASY
MEDIUM
[ Specific heat of water Latent heat of water ]
MEDIUM
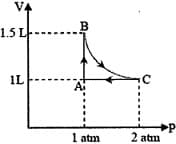
HARD

The correct option(s) is (are)
EASY
EASY
MEDIUM

EASY
EASY
(Latent heat of ice is and )
MEDIUM
[Heat of fusion of ice ; Specific heat of water ]
MEDIUM
(R = 8.314 J/mol K) (ln7.5 = 2.01)
EASY
MEDIUM
MEDIUM
HARD
EASY
HARD
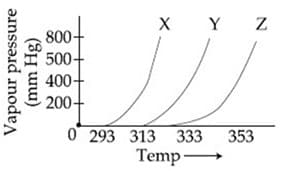
The following inferences are made:
has higher intermolecular interactions compared to
has lower intermolecular interactions compared to
has lower intermolecular interactions compared to
The correct inferences is/are:

