EASY
JEE Main
IMPORTANT
Earn 100
The nuclide contains (a) how many protons and (b) how many neutrons?

Important Questions on Nuclear Physics
EASY
JEE Main
IMPORTANT
EASY
JEE Main
IMPORTANT
HARD
JEE Main
IMPORTANT
Consider the three formation processes shown for the compound nucleus in the figure below. Here are some of the atomic and particle masses:
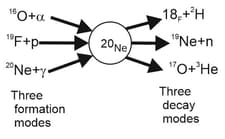
What energy must (a) the alpha particle, (b) the proton and (c) the -ray photon have to provide of excitation energy to the compound nucleus?
HARD
JEE Main
IMPORTANT
(Consider only the helium produced directly by plutonium and not by any by-products of the decay process)
EASY
JEE Main
IMPORTANT
EASY
JEE Main
IMPORTANT
EASY
JEE Main
IMPORTANT
EASY
JEE Main
IMPORTANT
