EASY
JEE Main
IMPORTANT
Earn 100
The number density of molecules of a gas depends on their distance r from the origin as, Then the numer of molecules is proportional to:
(a)
(b)
(c)
(d)
33.33% studentsanswered this correctly

Important Questions on Kinetic Theory of Gases
MEDIUM
JEE Main
IMPORTANT
A gas molecule of mass at the surface of the earth has kinetic energy equivalent to . If it were to go up straight without colliding with any other molecules, how high would it rise? Assume that the height attained is much less than the radius of the earth. ( is Boltzmann constant)
EASY
JEE Main
IMPORTANT
Modern vacuum pumps can evacuate a vessel down to a pressure of atm. at room temperature (300 K). Taking R = 8.3 JK-1 mole-1, 1 atm = 105 Pa and , the mean distance between molecules of gas in an evacuated vessel will be of the order of :
MEDIUM
JEE Main
IMPORTANT
of Nitrogen is enclosed in a vessel at a temperature of . The amount of heat required to double the speed of its molecules is_____ .
(Take )
EASY
JEE Main
IMPORTANT
The relation between root mean square speed and most probable speed for the molar mass of oxygen gas molecule at the temperature of will be
EASY
JEE Main
IMPORTANT
When a gas filled in a closed vessel is heated by raising the temperature by , its pressure increases by . The initial temperature of the gas is _____ .
EASY
JEE Main
IMPORTANT
A flask contains argon and oxygen in the ratio of in mass and the mixture is kept at . The ratio of their average kinetic energy per molecule respectively
EASY
JEE Main
IMPORTANT
A mixture of hydrogen and oxygen has volume , temperature , pressure and mass . The ratio of number of moles of hydrogen to number of moles of oxygen in the mixture will be
[Take gas constant ]
EASY
JEE Main
IMPORTANT
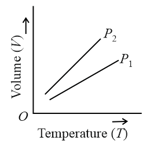
For a perfect gas, two pressures and are shown in figure. The graph shows