MEDIUM
MHT-CET
IMPORTANT
Earn 100
The number of de-Broglie wavelengths contained in the second Bohr orbit of the hydrogen atom is.
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Atoms, Molecules and Nuclei
EASY
MHT-CET
IMPORTANT
Match List-I (Fundamental Experiment) with List-II (its conclusion) and select the correct option from the choices given below the list:
| List-I | List-II | ||
| (a) | Franck-Herfz experiment | (i) | Particle nature of light |
| (b) | Photo-electric experiment | (ii) | Discrete energy levels of atom |
| (c) | Davison-Germer experiment | (iii) | Wave nature of electron |
| (iv) | Structure of atom |
EASY
MHT-CET
IMPORTANT
Two radioactive materials, and have decay constants and , respectively. If initially, they have the same number of nuclei, then the ratio of the number of nuclei of to that of will be after time equal to,
HARD
MHT-CET
IMPORTANT
An electron in the ground state of a hydrogen atom is revolving in anticlockwise direction in the circular orbit of radius . The atom is placed in a uniform magnetic induction such that the plane normal of the electron orbit makes an angle with the magnetic induction. The torque experienced by the electron is,
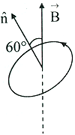
HARD
MHT-CET
IMPORTANT
What amount of energy should be added to an electron to reduce its de-Broglie wavelength from to ?
MEDIUM
MHT-CET
IMPORTANT
In the process of nuclear fission of uranium, the mass lost is milligram. The efficiency of power station run by it is . To obtain megawatt. power from the power station, the uranium required per hour is
EASY
MHT-CET
IMPORTANT
Assertion: Lyman series lies in the visible region of electromagnetic spectrum.
Reason: This is because Balmer series also lies in visible region.
MEDIUM
MHT-CET
IMPORTANT
The average life time of a hydrogen atom excited to state is . The period of revolution of the electron on an average before it jumps to ground state is,
EASY
MHT-CET
IMPORTANT
The binding energy per nucleon for and are and , respectively. The energy released when two combine to form is,
