EASY
9th CBSE
IMPORTANT
Earn 100
The number of neutrons and protons present in the nuclei of two atomic species A and B are given below.
Atomic Species
A
B
Protons
Neutrons
Write the mass numbers of A and B.
Important Questions on Structure of the Atom
EASY
9th CBSE
IMPORTANT
| Atomic Species | A | B |
|---|---|---|
| Protons | ||
| Neutrons | ||
What is the relation between two species?
EASY
9th CBSE
IMPORTANT
| Atomic Species | A | B |
|---|---|---|
| Protons | ||
| Neutrons |
Write the electronic configuration of atoms A and B
MEDIUM
9th CBSE
IMPORTANT
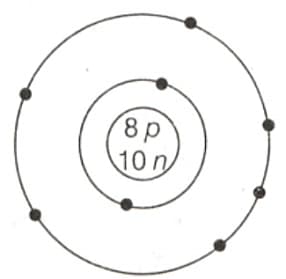
EASY
9th CBSE
IMPORTANT
What is such a particle called?
EASY
9th CBSE
IMPORTANT
What is the mass number of .
EASY
9th CBSE
IMPORTANT
What is the atomic number of .
MEDIUM
9th CBSE
IMPORTANT
EASY
9th CBSE
IMPORTANT
Calculate the mass number of the element.
