The number of properties which are state functions among the following is
Pressure, Volume, Temperature, Heat, Work, Entropy, Enthalpy, Free energy, Internal energy.

Important Questions on Thermodynamics
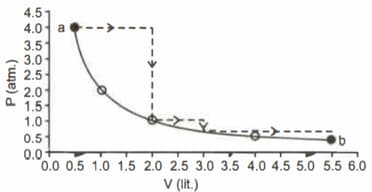
One mole of an ideal gas is taken from along two paths denoted by the solid and the dashed lines as shown in the graph below. If the work done along the solid line path is and that along the dotted line path is , then the integer closest to the ratio is
The surface of copper gets tarnished by the formation of copper oxide. gas was passed to prevent the oxide formation during heating of copper at . However, the gas contains of water vapour as impurity. The water vapour oxidise copper as per the reaction given below :
is the minimum partial pressure of (in bar) needed to prevent the oxidation at . The value of
is...........
(Given : total pressure= , (universal gas constant) = , and are mutually immiscible
At :
is Gibbs energy)
