The number of species having non-pyramidal shape among the following is:

Important Questions on Chemical Bonding and Molecular Structure
Match List - I with List - II:
| List-I (Species) | List-II (Number of lone pairs of electrons on the central atom) | ||
| (a) | (i) | ||
| (b) | (ii) | ||
| (c) | (iii) | ||
| (d) | (iv) | ||
Choose the most appropriate answer from the options given below:
Match List- with List-.
| List- | List- | ||
| (Molecule) | (Bond order) | ||
| (a) | (i) | ||
| (b) | (ii) | ||
| (c) | (iii) | ||
| (d) | (iv) | ||
Choose the correct answer from the options given below:
Given below are two statements:
Statement : -Nitrophenol is steam volatile due to intramolecular hydrogen bonding.
Statement : -Nitrophenol has high melting due to hydrogen bonding.
In the light of the above statements, choose the most appropriate answer from the options given below:
Given below are two statements: one is labelled as Assertion and the other is labelled as Reason .
Assertion : Dipole-dipole interactions are the only non-covalent interactions, resulting in hydrogen bond formation.
Reason : Fluorine is the most electronegative element and hydrogen bonds in are symmetrical.
In the light of the above statements, choose the most appropriate answer from the options given below:
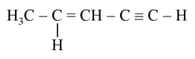 is _______.
is _______.